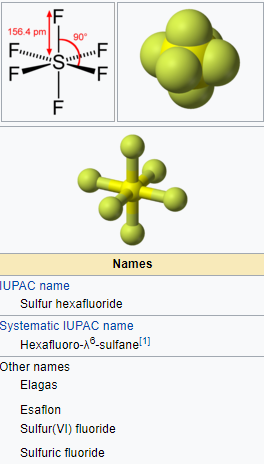
Sulfur hexafluoride (SF6) or Sulphur hexafluoride is an extremely potent and persistent greenhouse gas that is primarily utilised as an electrical insulator and arc suppressant. It is inorganic, colorless, odorless, non-flammable, and non-toxic. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.
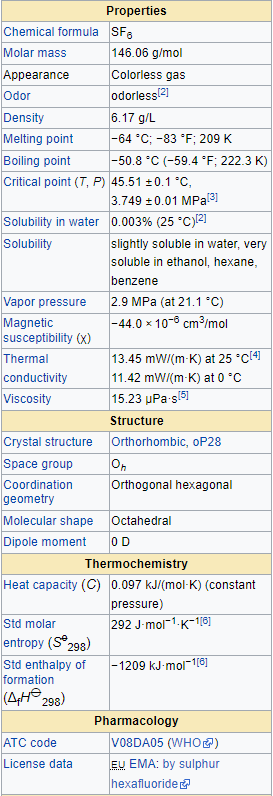
Typical for a nonpolar gas, SF6 is poorly soluble in water but quite soluble in nonpolar organic solvents. It has a density of 6.12 g/L at sea level conditions, considerably higher than the density of air (1.225 g/L). It is generally transported as a liquefied compressed gas.
The concentration of SF6 in Earth’s troposphere reached 10.63 parts per trillion (ppt) in 2021, rising at 0.36 ppt/year. The increase over the prior 40 years was driven in large part by the expanding electric power sector, including fugitive emissions from banks of SF6 gas contained in its medium- and high-voltage switchgear. Uses in magnesium, aluminium, and electronics manufacturing also hastened atmospheric growth.
Sulfur hexafluoride on Earth exists primarily as a man-made industrial gas, but has also been found to occur naturally
SF6 can be prepared from the elements through exposure of S8 to F2. This was also the method used by the discoverers Henri Moissan and Paul Lebeau in 1901. Some other sulfur fluorides are cogenerated, but these are removed by heating the mixture to disproportionate any
S2F10 (which is highly toxic) and then scrubbing the product with NaOH to destroy remaining SF4.
Alternatively, utilizing bromine, sulfur hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures (e.g. 100 °C), as follows:
2CoF3 + SF4 + [Br2] → SF6 + 2 CoF2 + [Br2]
There is virtually no reaction chemistry for SF6. A main contribution to the inertness of SF6 is the steric hindrance of the sulfur atom, whereas its heavier group 16 counterparts, such as SeF6 are more reactive than SF6 as a result of less steric hindrance (See hydrolysis example). It does not react with molten sodium below its boiling point, but reacts exothermically with lithium.
Table of Contents
Applications:-
The electrical power industry used about 80% of the sulfur hexafluoride produced in 2000, mostly as a gaseous dielectric medium. Other main uses as of 2015 included a silicon etchant for semiconductor manufacturing, and an inert gas for the casting of magnesium.
Dielectric medium:-
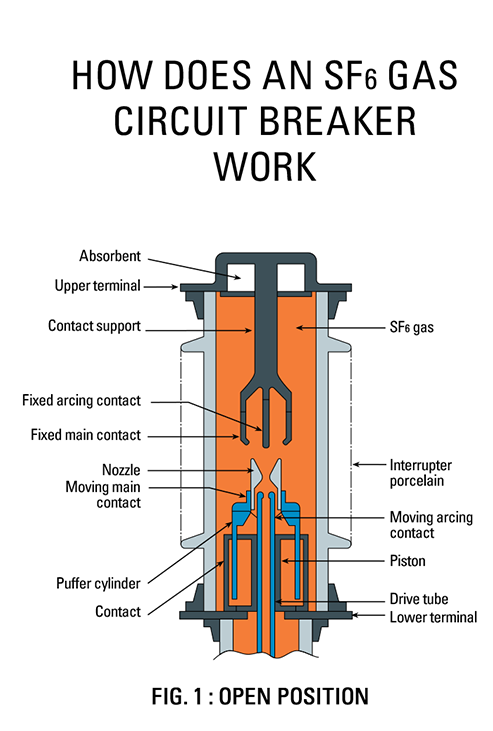
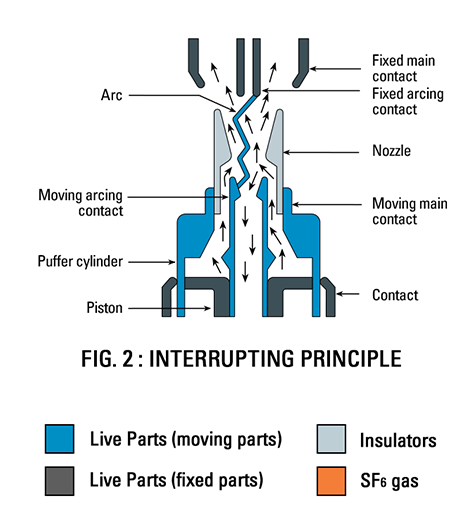
SF6 is used in the electrical industry as a gaseous dielectric medium for high-voltage sulfur hexafluoride circuit breakers, switchgear, and other electrical equipment, often replacing oil-filled circuit breakers (OCBs) that can contain harmful polychlorinated biphenyls (PCBs). SF6 gas under pressure is used as an insulator in gas insulated switchgear (GIS) because it has a much higher dielectric strength than air or dry nitrogen. The high dielectric strength is a result of the gas’s high electronegativity and density. This property makes it possible to significantly reduce the size of electrical gear. This makes GIS more suitable for certain purposes such as indoor placement, as opposed to air-insulated electrical gear, which takes up considerably more room.
Gas-insulated electrical gear is also more resistant to the effects of pollution and climate, as well as being more reliable in long-term operation because of its controlled operating environment. Exposure to an arc chemically breaks down SF6 though most of the decomposition products tend to quickly re-form SF6, a process termed “self-healing”. Arcing or corona can produce disulfur decafluoride (S2F10), a highly toxic gas, with toxicity similar to phosgene. S2F10 was considered a potential chemical warfare agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure.
SF6 is also commonly encountered as a high voltage dielectric in the high voltage supplies of particle accelerators, such as Van de Graaff generators and Pelletrons and high voltage transmission electron microscopes.Look up fluoroketone in Wiktionary, the free dictionary.
Alternatives to SF6 as a dielectric gas include several fluoroketones. Compact GIS technology that combines vacuum switching with clean air insulation has been introduced for a subset of applications up to 420 kV.
Medical use:-
SF6 is used to provide a tamponade or plug of a retinal hole in retinal detachment repair operationsin the form of a gas bubble. It is inert in the vitreous chamber and initially doubles its volume in 36 hours before being absorbed in the blood in 10–14 days.
SF6 is used as a contrast agent for ultrasound imaging. Sulfur hexafluoride microbubbles are administered in solution through injection into a peripheral vein. These microbubbles enhance the visibility of blood vessels to ultrasound. This application has been used to examine the vascularity of tumours. It remains visible in the blood for 3 to 8 minutes, and is exhaled by the lungs.
Other uses:-
- The magnesium industry uses SF6as an inert “cover gas” to prevent oxidation during casting. Once the largest user, consumption has declined greatly with capture and recycling.
- Insulated glazing windows have used it as a filler to improve their thermal and acoustic insulation performance.
- SF6 plasma is used in the semiconductor industry as an etchant in processes such as deep reactive-ion etching. A small fraction of the SF6 breaks down in the plasma into sulfur and fluorine, with the fluorine ions performing a chemical reaction with silicon.
- Tires filled with it take longer to deflate from diffusion through rubber due to the larger molecule size.
- Nike likewise used it to obtain a patent and to fill the cushion bags in all of their “Air”-branded shoes from 1992 to 2006. 277 tons was used during the peak in 1997.
- The United States Navy’s Mark 50 torpedo closed Rankine-cycle propulsion system is powered by sulfur hexafluoride in an exothermic reaction with solid lithium.
- Waveguides in high-power microwave systems are pressurized with it. The gas electrically insulates the waveguide, preventing internal arcing.
- Electrostatic loudspeakers have used it because of its high dielectric strength and high molecular weight.
- The chemical weapon disulfur decafluoride is produced with it as a feedstock.
- For entertainment purposes, when breathed, SF6 causes the voice to become significantly deeper, due to its density being so much higher than air, as seen in this video. This is related to the more well-known effect of breathing low-density helium, which causes someone’s voice to become much higher. Both of these effects should only be attempted with caution as these gases displace oxygen that the lungs are attempting to extract from the air. Sulfur hexafluoride is also mildly anesthetic.
Greenhouse gas:-
According to the Intergovernmental Panel on Climate Change, SF6 is the most potent greenhouse gas that has been evaluated, with a global warming potential of 23,900 times that of CO2 when compared over a 100-year period. Sulfur hexafluoride is inert in the troposphere and stratosphere and is extremely long-lived, with an estimated atmospheric lifetime of 800–3,200 years.
Physiological effects and precautions:-
Sulfur hexafluoride is a nontoxic gas, but by displacing oxygen in the lungs, it also carries the risk of asphyxia if too much is inhaled. Since it is more dense than air, a substantial quantity of gas, when released, will settle in low-lying areas and present a significant risk of asphyxiation if the area is entered. That is particularly relevant to its use as an insulator in electrical equipment since workers may be in trenches or pits below equipment containing SF6.
As with all gases, the density of SF6 affects the resonance frequencies of the vocal tract, thus changing drastically the vocal sound qualities, or timbre, of those who inhale it. It does not affect the vibrations of the vocal folds. The density of sulfur hexafluoride is relatively high at room temperature and pressure due to the gas’s large molar mass. Unlike helium, which has a molar mass of about 4 g/mol and pitches the voice up, SF6 has a molar mass of about 146 g/mol, and the speed of sound through the gas is about 134 m/s at room temperature, pitching the voice down. For comparison, the molar mass of air, which is about 80% nitrogen and 20% oxygen, is approximately 30 g/mol which leads to a speed of sound of 343 m/s.
Sulfur hexafluoride has an anesthetic potency slightly lower than nitrous oxide; it is classified as a mild anesthetic