Laws of Thermodynamics deals with the physical quantities which include entropy, energy, and temperature. These fundamental quantities play an important role in the thermodynamics systems which attained thermal equilibrium. So, the laws of thermodynamics tell us how these quantities will act in different situations.
There is a total of four laws Of thermodynamics as given below.
- Zeroth Law Of Thermodynamics
- The First Law Of Thermodynamics
- The Second Law Of Thermodynamics
- The Third Law Of Thermodynamics
Now we will understand each law one by one.
Table of Contents
1. Zeroth Law Of Thermodynamics:
To understand zeroth law, we will take the example of boiling water. Let’s say we have two cups namely A and B of the same volume which is filled with water. Now start boiling water on both cups. We know that water boils at 100-degree Celsius. So, keep heating both cups until it reaches 100-degree Celsius. Now take a thermometer and placed it into cup A. You will see the thermometer showing a 100-degree Celsius reading. So, we can say that the thermometer is in equilibrium with cup A. Now do the same thing in cup B and again thermometer will show 100-degree Celsius reading. That means the thermometer is in equilibrium with cup B also. Since the thermometer is in equilibrium with both the cups. We can say that cup A and cup B are also in equilibrium with each other. That’s what exactly the zeroth law of thermodynamics says.
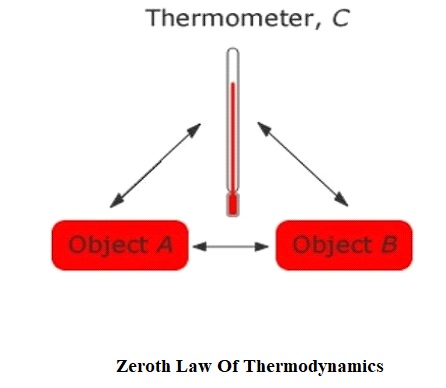
So, in simple words, we can say that according to the zeroth law of thermodynamics if two bodies are separately in equilibrium with a third body that means these first two bodies are also in equilibrium with each other.
For example – Let’s say body A and body B are separately in equilibrium with body C. That means according to zeroth law both body A and body B will also be in equilibrium with each other.
2. The First Law Of Thermodynamics:
The first law of thermodynamics works on the principle of the law of conservation of energy. It is also known by this name. According to the first law of thermodynamics, energy can neither be created nor can be destroyed. It can only be transferred from one form to another form.
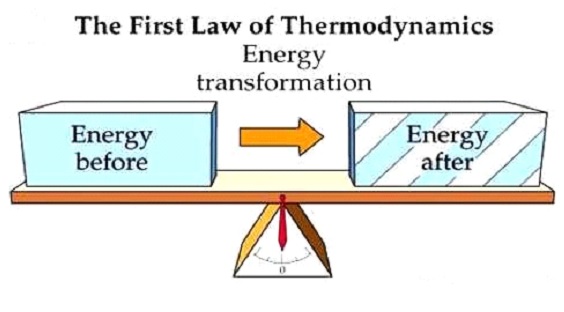
So now we will take some practical examples to understand this. The trees take sunlight and convert it into food or chemical energy through photosynthesis is a prime example of the first law of thermodynamics. Whenever we switch on the light the electric bulbs convert electrical energy into light energy is another example of the first law of thermodynamics.
3. The Second Law Of Thermodynamics:
The second law of thermodynamics deals with the disorderliness of a system and the universe. We call this disorderliness or randomness entropy.
So according to the second law of thermodynamics, the entropy of a system that is isolated will always increase. If a system is isolated, then it will spontaneously move towards a thermal equilibrium which is the maximum entropy state of the system. It also states that the entropy or disorderliness of the universe will always increase rather than decrease. It also includes Kelvin – Plank’s statement and Clausius – statement.
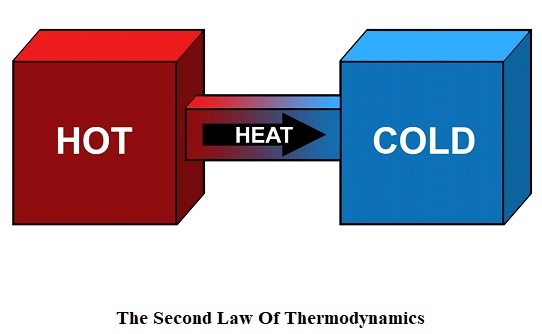
For example, if a room is properly organized that means it has less disorderliness. Which means less entropy. But after some time, it got messy and become disorganized which means it has high disorderliness. While means high entropy. Even if you clean the room again, the room disorderliness will decrease but whatever you have done during the cleaning process will increase the entropy or disorderliness outside. That’s why universe entropy will always increase.
4. The Third Law Of Thermodynamics:
The third law of thermodynamics gives a relation between the entropy of a system to its temperature.
According to the third law of thermodynamics, the entropy of the system will proceed towards a constant value when the temperature of that system advances towards absolute zero.
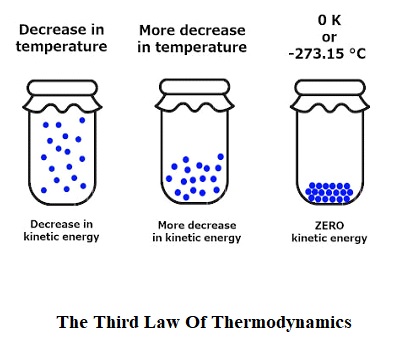
To understand the third law of thermodynamics we will take the example of steam. In the steam form, the molecules will move freely without any restrictions. so, it will produce more disorders. That means steam has high entropy. Now if we decrease the temperature of steam below 100-degree Celsius then it will become water. This time molecules will move but not as free as they used to move in the steam So that means less disorderliness. While means entropy will also decrease. Now if we decrease the temperature of the water below 0-degree Celsius and convert it into ice. So, in this case, molecules’ movement will be restricted. That means it will have less entropy compared to both water and steam.
From this pattern, we can understand that as we are decreasing the temperature and advancing towards absolute zero its entropy is also decreasing. If somehow, we will able to bring down the ice at absolute zero (ideally) then the entropy will also become zero. But in reality, it is not possible to bring down any object or a system to absolute zero.
If you like my article on the laws of thermodynamics, please let me know.
