The preservation of stones is crucial to prevent their decay, and different types of stones require specific treatments.

Generally, stones should be dried with the use of a blow lamp before applying a protective coating such as paraffin, linseed oil, or light paint onto the surface. However, it should be noted that this treatment is periodic and not permanent. For example, when treating stones with linseed oil, it is boiled and then applied in three coats over the stone.
Afterward, a coat of dilute ammonia in warm water can be applied to further enhance the preservation process. It’s essential to understand the appropriate treatment method for each type of stone to ensure its longevity and durability.
To maintain the structure of stones and preserve them, it is important to clean them frequently with water and steam to remove dirt and deposited salts. However, the best way to preserve them is by applying preservatives. One such method is to wash the stones with a thin solution of silicate of soda or potash, followed by the application of a solution of CaCl2 after drying. These two solutions, also known as Szerelmy’s liquid, react to form silicate of lime, which fills the pores in the stones. The common salt formed during the process is washed away, and the resulting silicate of lime forms an insoluble film that protects the stones.
While lead paint is sometimes used to preserve stones, it alters their natural color. Another method is to paint stones with coal tar, but this can also spoil their beauty. It is best to avoid using chemicals, especially caustic alkalis, as they may introduce salts that could cause damage to the stones. Although chemical cleaning may be easy, it poses a risk to the stones’ long-term preservation.
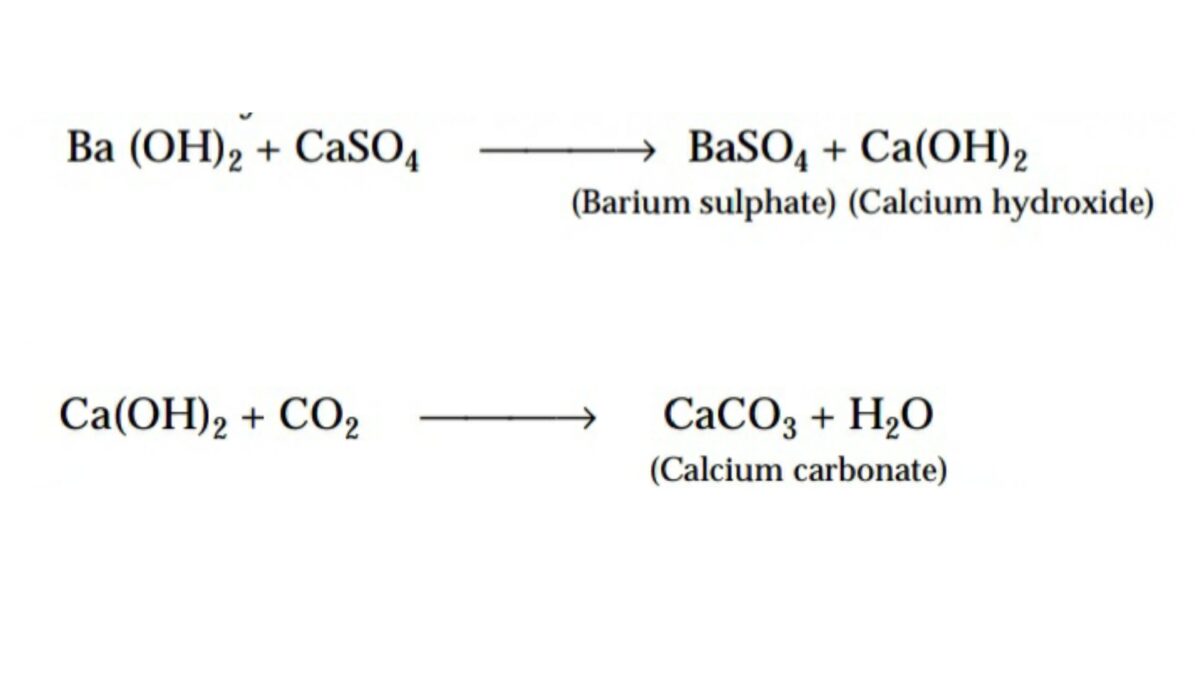
The application of a solution of baryta, Ba(OH)2 – barium hydrate, preserves stones in industrial towns. The sulphur dioxide present in the acid reacts with the calcium content of the stones, forming calcium sulphate. Soot and dust in the atmosphere adhere to the calcium sulphate, forming a hard skin. Over time, the calcium sulphate flakes off, exposing fresh stone surface for further attack. This process is known as sulphate attack. Baryta reacts with the calcium sulphate deposited on the stones and forms insoluble barium sulphate and calcium hydroxide. Carbon dioxide from the air is absorbed by the calcium hydroxide, forming calcium carbonate.
It is a complex issue to determine whether or not to use stone preservatives on old and deteriorated stone. The effectiveness of various treatments is challenging to assess based on concrete evidence. Although these treatments, when applied with care in favorable conditions, may appear to slow down the decay rate, the slow rate of stone decay means that a short period of observation is insufficient to establish their effectiveness.
Some treatments that seem successful for a few years may ultimately fail to maintain the improvement. In fact, the benefits of preservatives are still uncertain, and they may even be harmful when evaluated over an extended period.
