Table of Contents
What is Surface Tension?
Surface tension is defined as the force per unit length acting at right angles to an imaginary line drawn on the free surface of a liquid.
In other words, surface tension can be defined as,
Surface tension is also defined as the tensile force acting on the surface of a liquid in contact with a gas or on the surface between two immiscible liquids, such that the contact surface behaves like a film under tension.
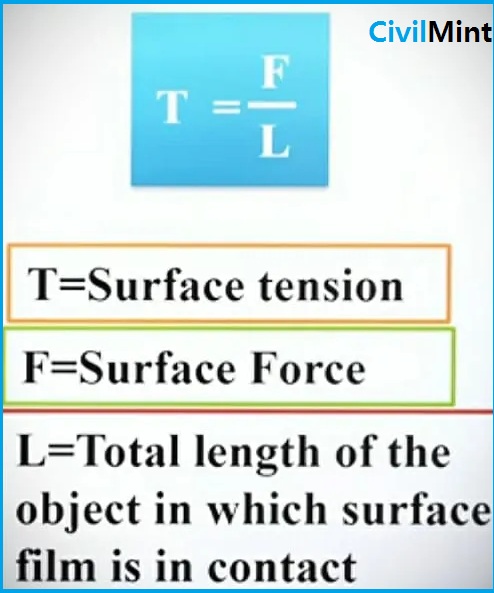
What is the Unit of Surface Tension?
Units for surface tension are denoted by the Greek letter (uppercase Σ, lowercase σ). However, lowercase sigma is used to denote surface tension.

Example of surface tension:
To better understand the phenomenon of surface tension we will understand it by an example given below.
Trampoline:
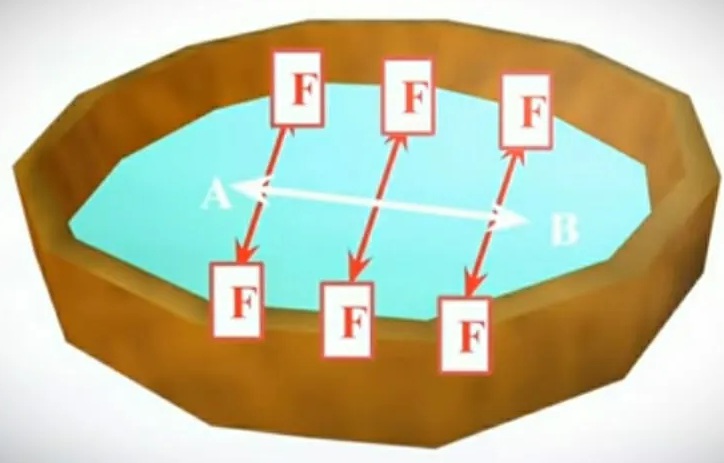
Let’s analyze this definition in detail with an example. Take the liquid to a bowl. Now draw an imaginary “AB” line on the liquid surface. As the definition implies, the surface force acts perpendicular or perpendicular to the imaginary line, and this force is called “F”.
So these surface forces are clearly visible in the diagram. These forces increase the liquid surface of the stretched membrane. These forces keep the surface of the liquid stretched. This stress on the surface of a liquid is called surface tension.
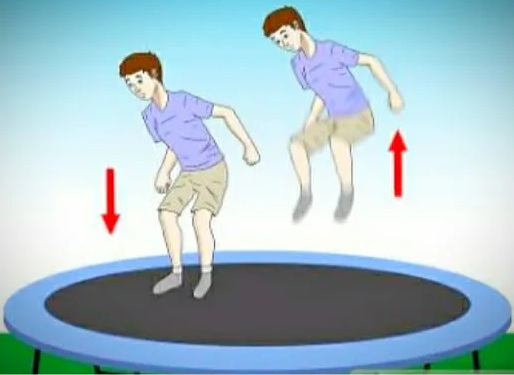
The best example of a stretch membrane is a trampoline. When looking at the surface of a trampoline, it tends to jump when someone jumps on the surface. It does not allow a person to fall to the ground. It just pushes people in the opposite direction.
If you find a rope attached to the edge of the trampoline. When a person jumps over the water, this spring stretches so that the person does not fall to the ground. Sleep behaves similarly to this example.
The surface of the water is in a state of tension. This will cause very light objects such as safety pins or mosquitoes to float on the surface of the water.
Effect of Impurities on the surface tension of water
When impurities are added to a liquid or water, the bonding properties between molecules are changed. As a result, the surface tension of the water also changes. Let’s take a closer look at this.
In general, I’ve seen governments spray kerosene on the surface of stagnant water whenever there is an increase in mosquitoes. This is because the kerosene is sprayed on the surface of the liquid. The surface tension of water decreases. A decrease in surface tension prevents mosquitoes from swimming on the surface of the water. As a result, playback stops.
In addition, the addition of sorbent, phenol, and detergent reduces the surface tension of the water. You may have noticed that when a drop hits the surface of the fabric, it doesn’t get wet and is due to the surface tension of the water. Therefore, washing clothes with soap and detergent can reduce the surface tension of the water.
However, adding salt to water increases the surface tension of the water.
Effect of temperature on the surface tension of water
The surface tension of water or liquid decreases with increasing temperature. Let’s take a closer look at this.
It is said that washing clothes in warm water usually washes clothes better than in cold water. As the water temperature rises, the surface tension of water decreases.
As the temperature rises, the average distance between molecules increases and, consequently, the cohesive force decreases. At a higher temperature, the surface tension of water reaches zero at a point, and this temperature is called the critical temperature of this liquid.
Importance of Surface Tension
Surface tension or interfacial tension is a very important phenomenon that plays a very important role in daily life. There are many examples where surface tension plays an important role.
Take, for example, a spider that walks on the surface of the water. This is possible due to surface tension. The surface tension of the water can support the weight of the spider. Change the water to ethanol and the poor spider will be sucked into the water. This is because the surface tension of ethanol is very low compared to that of water.
Surface tension is the only reason waterfalls a spherical rain. Generally, the shape of water is spherical. This is because water tends to shrink in the area. We can understand this example.
For a specific area where the object should be designed. Try different shapes but the smallest shaped object with the same area will be a spherical-shaped object. So due to this water always takes a spherical shape.
But now you might think that spiders aren’t that important, they can survive on land, and the shape of the water isn’t important enough to worry about either. However, the surface tension of water will be much lower than it is today.
Then nothing will float on the surface of the water. Everything, even the smallest particles on Earth, will sink to the bottom, destroying the ecosystem. Ultimately, water will never be in liquid form, it will evaporate into the atmosphere, and there will be no life on Earth.
By now I think it’s pretty obvious, and I’m pretty sure that surface tension is important for the existence of life on the Earth’s surface.
Surface tension tests
- Capillary rise method.
- Drop method.
- Torsion balance method.
1. Capillary rise method:
To understand the concept of capillary rise, let’s first understand the concept of contact angle.
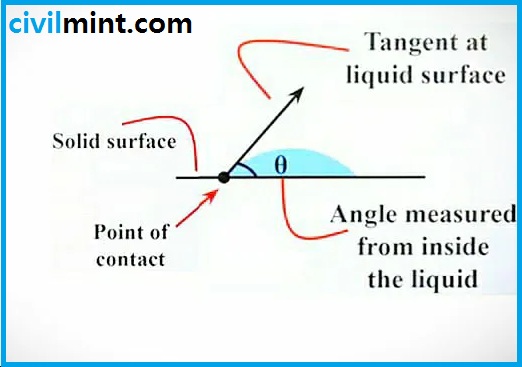
Angle Of Contact:
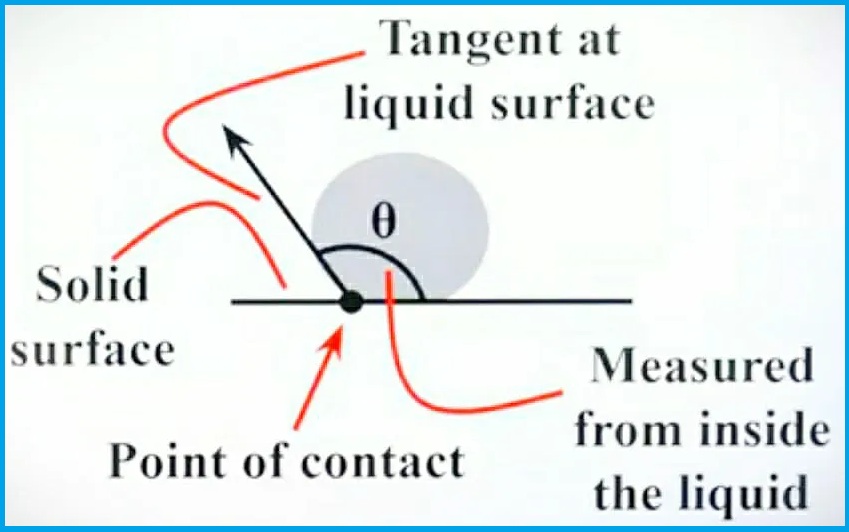
You may have noticed that the drops on the leaves are round after the rain has stopped, and pouring a drop of water on the surface of glass gives an example of a drop of mercury in a different state than the glass., it goes like this. different form. See the diagram below to understand this.
Case 1:
In the first case, you can see that the water droplets on the glass surface spread better than the mercury. In the case of water, the adhesive force is greater than the cohesive force. In other words, it can be said that adhesion dominates over cohesion. It attracts or sticks to the glass surface because of its greater adhesion in water.
So, a drop of water will look like the picture. So we study the contact angle of water and glass. First, you need to draw a tangent to the water surface and then measure the angle between the tangent to the droplet and the glass surface. So you get the contact angle of glass and water. “θ”.
Here, we learned that with respect to fluid cohesion, the greater the fluid adhesion, the sharper the contact angle. Those. Contact angle less than 90°.
Case 2:
In the second case, you can see that a drop of mercury on the surface of the glass does not spread compared to the water. Mercury is more cohesive with respect to adhesion. In other words, it can be said that cohesion dominates adhesion. Since mercury is more cohesive, it is not attracted to the glass surface.
Therefore, a drop of mercury has the shape of a picture. So, in the case of mercury and glass, investigate the contact angle. You must first draw a tangent to the surface of the mercury and then measure the angle between the tangent to the drop of mercury and the surface of the glass. Thus, the contact angle of glass and mercury can be obtained. “θ”.
Here we learned that with respect to fluid adhesion, the greater the fluid cohesion, the duller the contact angle. i.e. a contact angle greater than 90°.
Now returning to the topic, we study the methods of capillary action.
What is Capillarity?
Capillary action is defined as the rise or fall of the surface of a liquid within a small tube relative to the level of the entire adjacent liquid when the tube is held perpendicular to the liquid.
There are two conditions for capillary action.
- Capillary rise.
- Capillary fall.
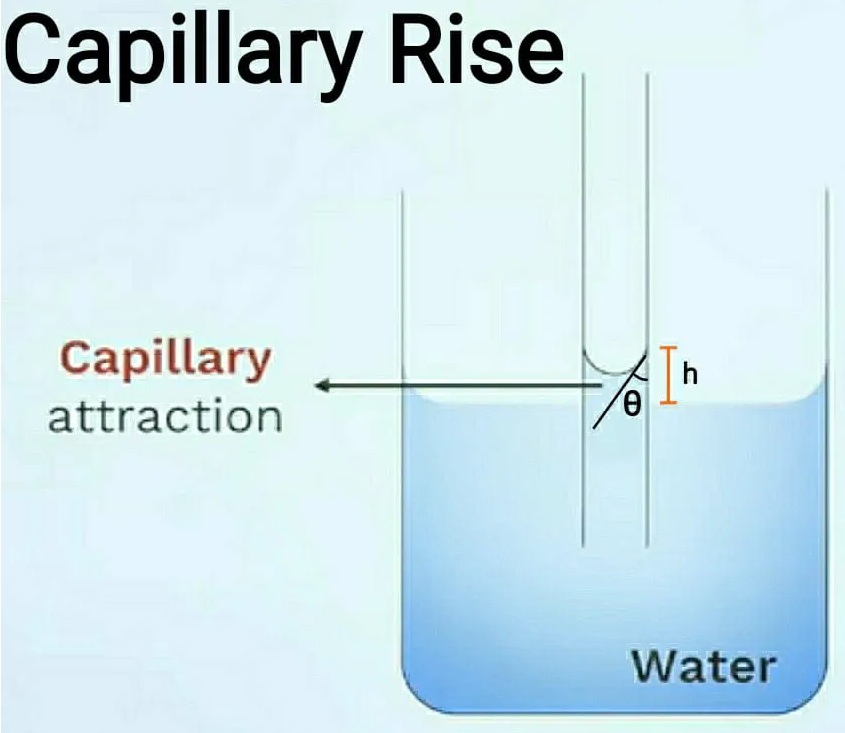
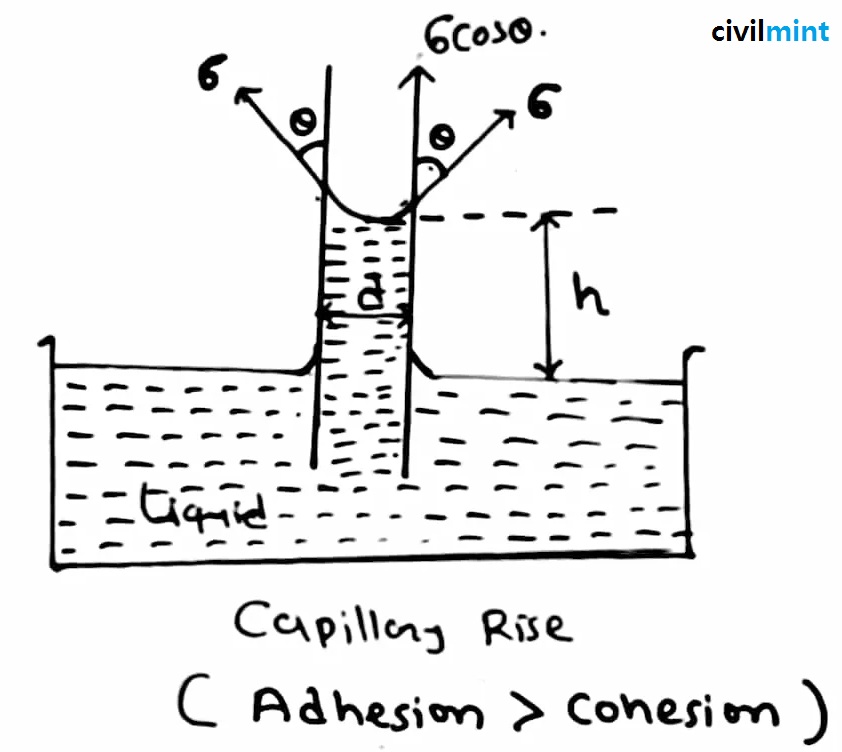
1. Capillary rise:
This condition is formed in any liquid in which the adhesive force is greater than the cohesive force. So, drink the liquid in a glass of water. Then take a very thin glass tube and place it in the liquid.
When lowered to a liquid, the water rises to a height “h”. As the water rises over the tube, a convex meniscus is formed at the top. This capillary rise is also known as capillary attraction. The formula for measuring the height “h” is given below, please refer to the chart for better understanding.
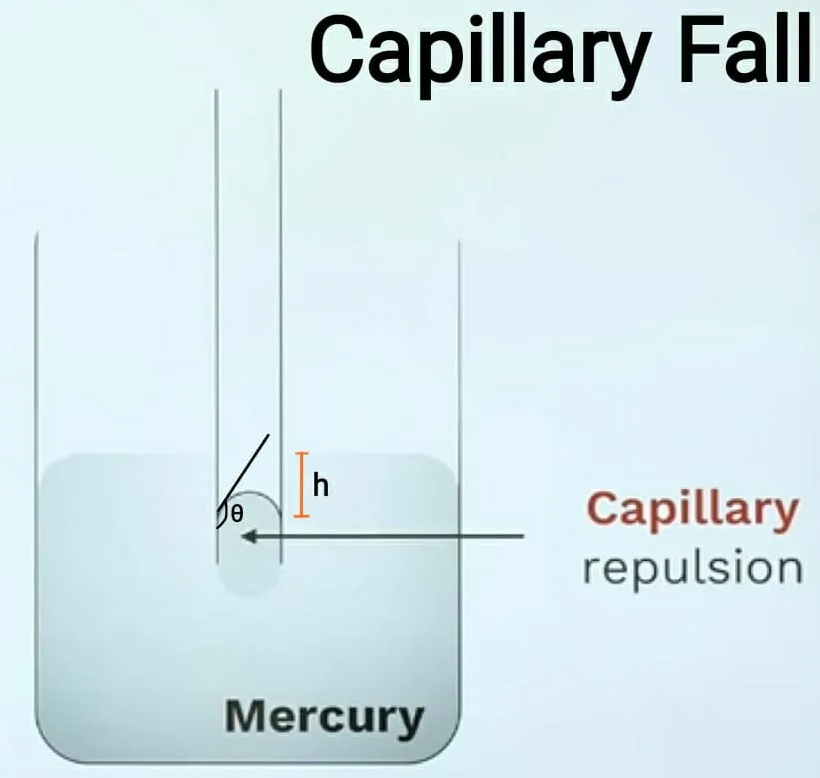
2. Capillary fall:
This state is formed in liquids such as mercury, where cohesion is greater than adhesion. So, take the liquid in a glass with mercury. Then, a very thin glass tube is placed in the mercury. When placed in mercury, the mercury level drops to “h” height. As the mercury falls into the tube, it forms a concave top. This capillary bubble is also called capillary repulsion. Please refer to the chart below to understand more, and the formula for measuring “h” height is also provided below.
To calculate the value of ‘h’ you can use the following formula:
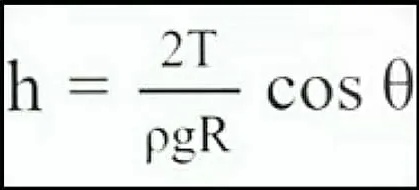
Where,
h = Height of rising or fall
T = Surface tension
g = Acceleration due to gravity
R = Radius of tube
ρ = Density of liquid
Θ = Angle of contact
2. Drop method:
A stalagmite system can be used to determine the relative surface tension of any liquid. This method is called drip method. This tool has a light bulb in the center of the tube. These bulbs are connected by two tubes at the top and the bottom. There are two markings on this tube. The tube at the top is marked “A” and the tube at the bottom is marked “B”. Take a look at the diagram below to learn more about this tube.
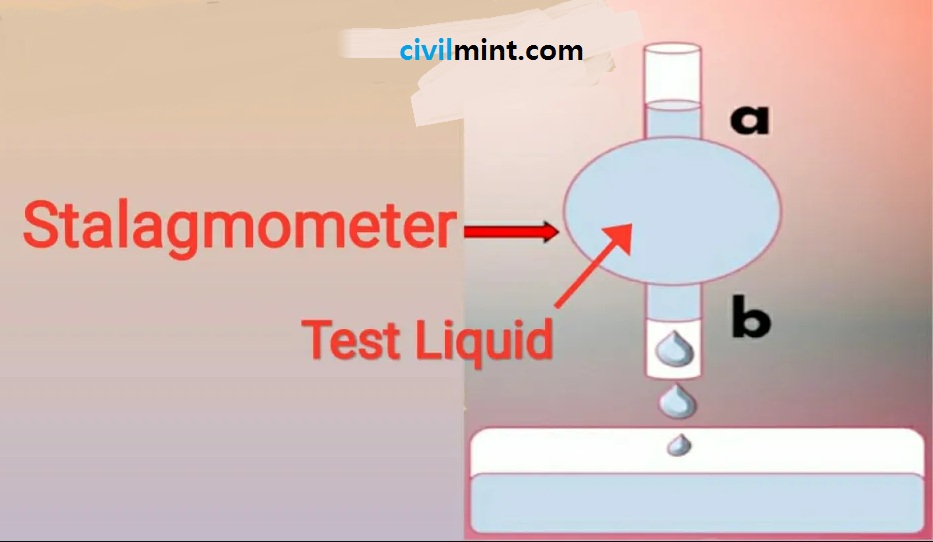
Test Process:
First, water is added to the tube from point “A” to point “B”. Then, a drop of water drips into the glass and the number of drops is recorded. After that, replace the water with the test solution and follow the same process. This procedure is performed 3 times. You can measure the relative surface tension using the formula:
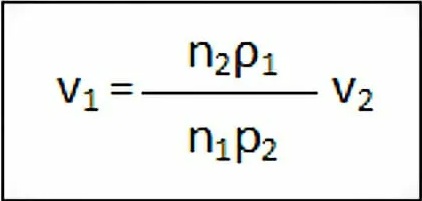
Where,
V1 = Surface tension of test liquid
V2 = Surface tension of water
ρ1 & ρ2 = Density of test liquid & water
n1 = No. of drops of test liquid
n2 = No. of drops of water
Surface Tension Measurement:
Derivation:

