Table of Contents
Introduction
Protein are chains of amino acids joined together by peptide bonds. Many confirmation or this chain are possible due to the rotation of the chain about each Cα atom. It is these confirmational changes that are possible for difference in the three diamensional structure or proteins.
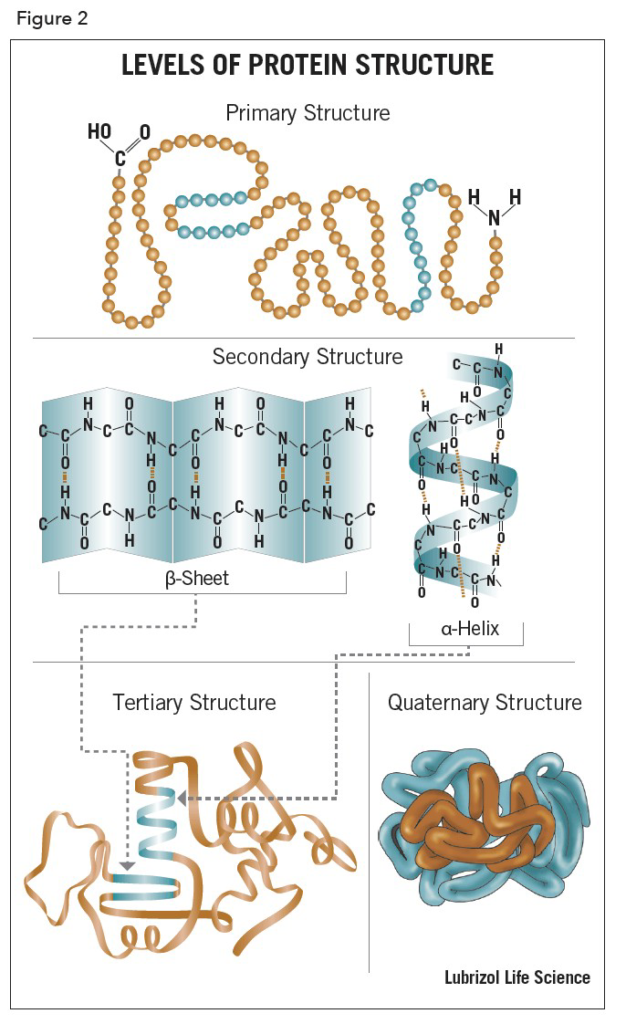
1. Primary Structure
The primary structure of a protein refers to the linear sequence or Ami no acids in the polypeptide chain. The primary structure is held together by covalent bonds such as peptid bonds, which are made during the process of protein biosynthesis or translation. The two ends or the polypeptide chain are referred to as the carboxyl terminus (C-terminus) and the amino terminus (N-terminus) based on the nature of the free group on each extermity.
The sequence of a protein is determined by the DNA of the gene that encodes the protein (or that encodes a portion of the protein, for multi-subunit proteins). A change in the gene’s DNA sequence may lead to a change in the amino acid sequence of the protein. Even changing just one amino acid in a protein’s sequence can affect the protein’s overall structure and function.

2. Secondry Structure (α helix)
The helix is the most abundant of secondry structure in proteins. The helix has 3.6 amino acids per turn with an H bond formed between every fourth residue. The α helix is stabilized by hydrogen bonds between an amide hydrogen of one amino acid and a carbonyl oxygen four amino acids

Secondary structure of the proteins can be used to predict the tertiary structure since predicting only with amino acid sequence may not be sufficient. The secondary structure of proteins is determined by the pattern of hydrogen bonding.
3. Tertiary Structure
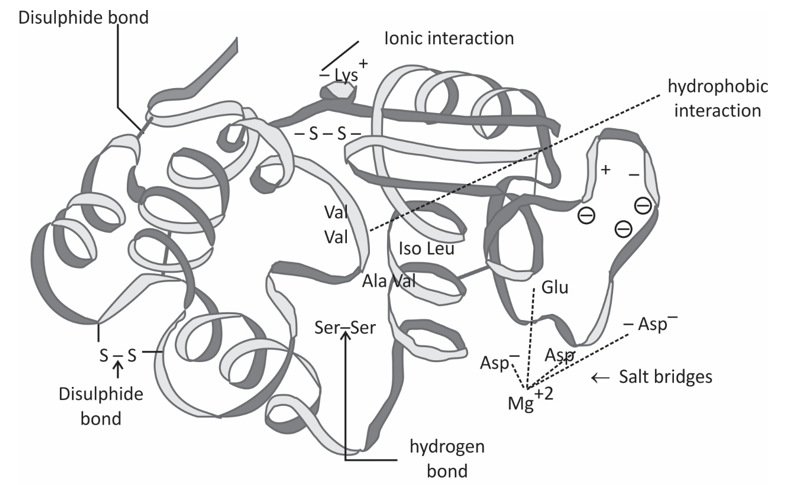
Tertiary structure refer three diamensional structure of a single, double or triple bonded protein molecule. The protein molecule will bend and twist in such a way as to achieve maximum stability or lowest energy state. Although the three-dimensional shape of a protein may seem irregular and random, it is fashioned by many stabilizing forces due to bonding interactions between the side-chain groups of the amino acids.
Under physiologic conditions, the hydrophobic side-chains of neutral, non-polar amino acids such as phenylalanine or isoleucine tend to be buried on the interior of the protein molecule, thereby shielding them from the aqueous medium. The alkyl groups of alanine, valine, leucine and isoleucine often form hydrophobic interactions between one another, while aromatic groups such as those of phenylalanine and tyrosine often stack together. Acidic or basic amino acid side-chains will generally be exposed on the surface of the protein as they are hydrophilic.
Difference Between Primary, Secondary and Tertiary Structure of Proteins
The main difference between primary secondary and tertiary structure of protein is that the primary structure of a protein is linear and the secondary structure of a protein can be either an α-helix or β-sheet whereas tertiary structure of a protein is globular.
