Table of Contents
Thermodynamic Process:
Thermodynamic Process can be defined as when some changes in the energy of a system take place. The changes in the energy of the system can happen due to changes in the volume, pressure, or Internal energy of a system.
There are four types Of thermodynamic processes as follows.
- Adiabatic Process
- Isochoric Process
- Isobaric Process
- Isothermal Process
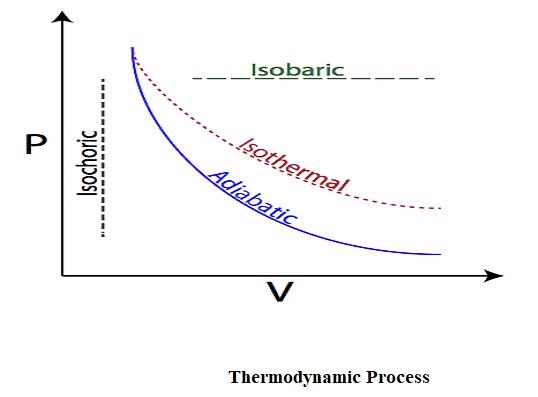
1. Adiabatic Process
Adiabatic process in thermodynamics can be defined as the process in which no heat transfer takes place. That means the heat can neither enter nor leave the system in an adiabatic process.
2. Isochoric Process
Isochoric process in thermodynamics can be defined as the process in which volume doesn’t change. That means the system does not work since its volume is constant.
3. Isobaric Process
Isobaric process in thermodynamics can be defined as the process in which there is no change in pressure takes place. That means the system pressure remains constant throughout the isobaric process.
4. Isothermal Process
Isothermal process in thermodynamics can be defined as the process in which there is no changes in temperature takes place. That means the temperature of the system remains constant throughout the isothermal process.
Thermodynamic Cycle
The thermodynamic cycle includes a number of processes which is conducted to keep the initial and final state of the system the same. It is also known by the name of cyclic process or operations.
Thermodynamic Properties
Thermodynamic properties define the state of a system. It provides characteristic features to specify the system. Without thermodynamic properties, it will be impossible to describe a system and its process.
Thermodynamic Properties Can Be Classified Into Two Categories-
- Extensive Properties
- Intensive Properties
1. Extensive Properties
The value of the extensive properties in thermodynamics depends on the mass of the system. Enthalpy, energy, and volume are good examples of extensive properties.
2. Intensive Properties
The value of intensive properties in thermodynamics does not depend on the mass of the system. That means intensive properties are independent of the quantity of matter. Pressure and temperature are good examples of intensive properties.
Enthalpy
In thermodynamics, the energy of a system is measured in enthalpy or we can say that the enthalpy of a system represents the total energy in it. So we can say that enthalpy which is denoted by H is equal to the sum of internal energy E and product of the pressure P and volume V of the system.
H = E + PV
H = Enthalpy
E = Internal Energy
P = Pressure in a system
V = Volume of a system
Entropy
Entropy in thermodynamics measure the disorderliness or randomness of a system. The value of entropy depends on the state in which the system is. For example – Steam has higher entropy than water because it has higher disorderliness or randomness of molecules compared to water. It is generally denoted by the letter S.
